Wrestling with SUMO to combat atherosclerosis
© 2014 EPFL
Atherosclerosis is a complex disease that develops from the interplay between hypercholesterolemia, dyslipidemia and chronic inflammation and encompasses several tissues and organs. The disease remains asymptomatic for decades, but eventually an atherosclerotic plaque may rupture, leading to atherothrombosis and in worst case causing a myocardial infarction or stroke, two of the primary causes of morbidity and mortality in the world.
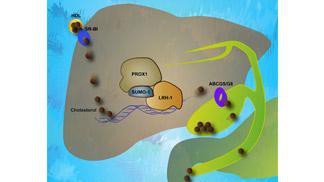
One hallmark of atherosclerosis is the accumulation of excessive cholesterol in the blood vessels. EPFL scientists have characterized the atherosclerotic potential of a non-SUMOylatable LRH-1 mouse model in collaboration with researchers from Groningen, Heidelberg and New York. According to their study, the nuclear receptor LRH-1 could be a novel pharmacological target to promote excretion of cholesterol from the body via the reverse cholesterol transport.
Earlier work led by the group of Kristina Schoonjans showed that LRH-1 regulates various metabolic processes in the liver, pancreas, ovary and intestine. This is the first study to show that the posttranscriptional modification of LRH-1 by SUMOylation also exerts important physiological effects.
In the present study, the EPFL team demonstrates that LRH-1 is a nuclear receptor whose activity is repressed by SUMO modification of 1 single lysine residue. Moreover, they show that atherosclerosis-prone mice carrying a mutation that abolishes the SUMOylation of the receptor are significantly protected from atherosclerotic lesion formation. The mechanism underlying this atheroprotection involves a local increase of reverse cholesterol transport in the liver and is secondary to a compromised interaction of the non-SUMOylatable form of LRH-1 with the co-repressor Prox-1.
The work, published in the current issue of the scientific journal Cell Metabolism (August 28, 2014), exposes a mechanistic basis of how SUMOylation of a nuclear receptor affects its selective interaction with cofactors and determines its transcriptional activity in a highly context-specific manner. In addition, the physiological consequences of LRH-1 SUMOylation on the development of atherosclerosis highlight the translational value of the study.
Contacts : Kristina Schoonjans, corresponding author, EPFL, +41 21 693 18 91, [email protected] - Sokrates Stein, first author, EPFL, +41 21 693 18 65, [email protected]