Undoped silicon makes simpler, cheaper but efficient solar cells
© PVLab-EPFL
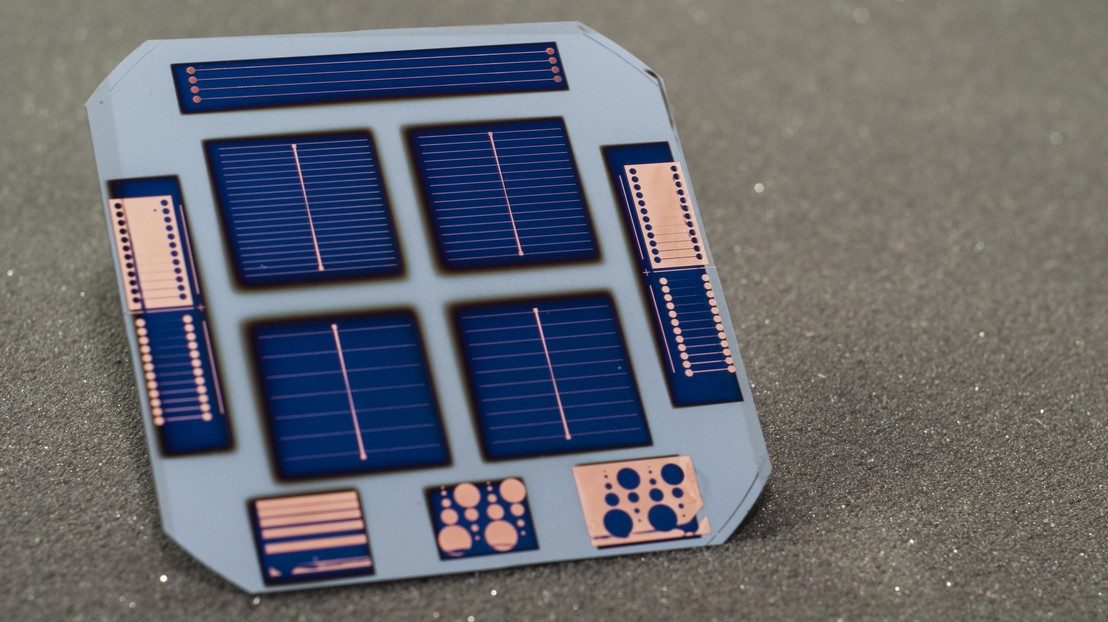
A group of international researchers led by Berkeley scientists create a high-efficiency silicon device in seven steps. The core part of the prototypes have been built at EPFL's Photovoltaics Lab, led by Christophe Ballif, in Neuchâtel.
Researchers have simplified the steps to create silicon solar cells that convert sunlight to electricity with high efficiency by applying a new mix of materials to a standard design. Published this week in Nature Energy, the work is a result of a collaboration between the Lawrence Berkeley National Laboratory (Berkeley Lab), EPFL, and the Australian National University (ANU).
The special blend of materials – that could also prove useful in semiconductor components – eliminates the need for a process called “doping”, which steers the device’s semiconductor properties by introducing foreign atoms, but also makes the device’s processing more complicated and causes losses in performance.
"The solar-cell industry is driven by the need to reduce costs and increase performance," says James Bullock, the lead author of the study. Bullock participated in the study as a visiting researcher from the ANU at the U.S. Department of Energy's Berkeley Lab and UC Berkeley, where he will return soon as a postdoc.
“Conventional silicon solar cells use impurity doping, which brings about a number of limitations that make progress increasingly difficult. If you look at the architecture of the solar cell we made, it is very simple. That simplicity can translate to reduced costs."
The majority of today’s solar cells use crystalline silicon wafers. The wafer itself, and sometimes layers deposited on the wafer, are doped with atoms that either have electrons to spare when they bond with silicon atoms, or alternatively generate electron deficiencies – so called "holes". In either case, doping enhances electrical conductivity.
These two types of dopant atoms are required at the electrical contacts to regulate the way that electrons and holes travel across a solar cell so that sunlight is efficiently converted to electrical current flowing out of the cell. However, doping itself can degrade its overall performance.
Crystalline silicon-based solar cells with doped contacts can exceed 20 percent power-conversion efficiency – meaning more than 20 percent of the sun's energy is converted to electricity. Dopant-free silicon cells had never exceeded 14 percent efficiency before. The new study, though, introduces a dopant-free silicon cell – referred to as a DASH cell (dopant free asymmetric heterocontact) – with an efficiency approaching 20 percent. The cell is made with new materials and a simple coating process for layers on the top and bottom of the device. The study also shows that it is possible to create a solar cell in only seven steps.
In this study, the research team used a crystalline silicon core (or wafer) and applied layers of dopant-free amorphous silicon for surface passivation. Then, they applied ultrathin coatings of a material called molybdenum oxide, also known as moly oxide, at the sun-facing side of the solar cell, and lithium fluoride at the bottom surface. The two layers act as dopant-free contacts for holes and electrons, respectively.
"Moly oxide and lithium fluoride exhibit extremely high and low work functions, respectively, which make them ideal for dopant-free electrical contacts,” says lead senior author Ali Javey, program leader of Electronic Materials at Berkeley Lab and a professor of Electrical Engineering and Computer Sciences at UC Berkeley. “They were previously explored for organic devices, but they were not carefully explored by the crystalline Si solar cell community." Javey notes that both materials are transparent, and have complementary electronic structures that are well-suited for solar cells.
Javey’s group discovered the utility of moly oxide as an efficient hole contact for crystalline silicon solar cells a couple of years ago. "It has a lot of defects, and these defects are critical and important for the arising properties,” he says. “These are good defects."
Stefaan de Wolf, team leader on crystalline silicon at EPFL Neuchâtel (Switzerland) adds: “We have adapted the technology in our solar-cell manufacturing platform at EPFL, and found out that these moly oxide layers work extremely well when optimized and used in combination with thin amorphous layers of silicon on crystalline wafers, allowing amazing variations of our standard heterojunction approach.”
In the current work, the team identified lithium fluoride as a good candidate for electron contacts to crystalline silicon that has been coated with a thin amorphous layer, thus complementing moly oxide hole contacts. The team used a room-temperature technique called “thermal evaporation” to deposit the layers of lithium fluoride and moly oxide for the new solar cell. There are many other materials that the researchers hope to test to see if they can improve the cell's efficiency.
According to Javey, there is also potential for adapting the material mix used here to improve the performance of semiconductor transistors. "There's a critical need to reduce the contact resistance in transistors, so we're trying to see if this can help."