Primates Regain Control of Paralyzed Limb
© 2016 Jemere Ruby / EPFL
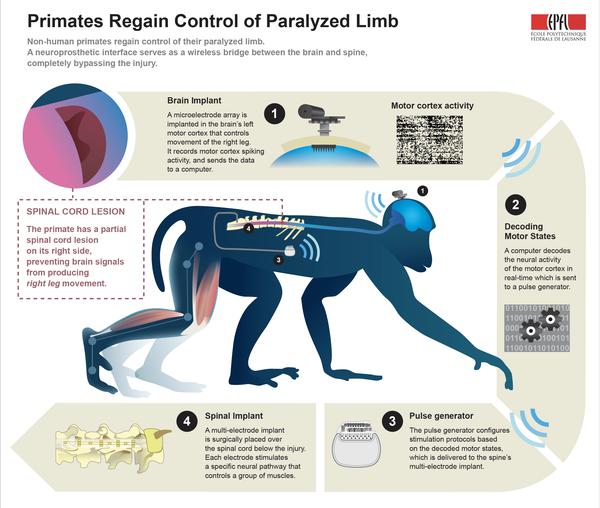
Non-human primates regain control of their paralyzed leg – as early as six days after spinal cord injury – thanks to a neuroprosthetic interface that acts as a wireless bridge between the brain and spine, bypassing the injury. A feasibility clinical study has begun in Switzerland to test the therapeutic effects of the spine-part of the interface in people with spinal cord injury.
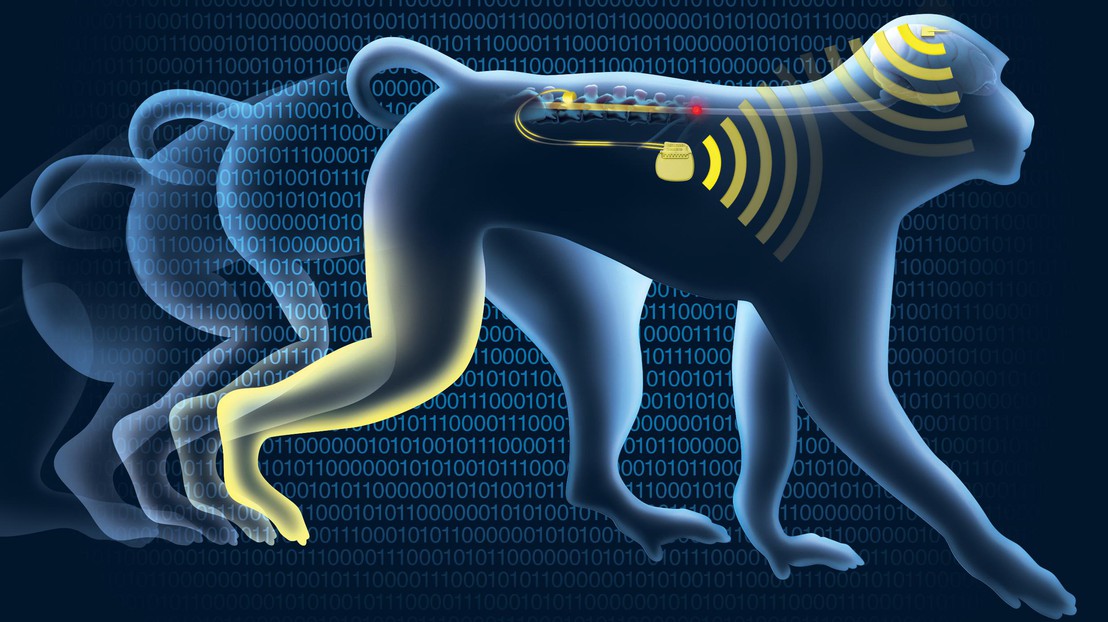
On June 23rd, 2015, a primate with spinal cord injury regained control of its paralyzed leg with the help of a neuroprosthetic system called the “brain-spine interface” that bypassed the lesion, restoring communication between the brain and the region of the spinal cord. The results are published today in Nature.
The interface decodes brain activity associated with walking movements and relays this information to the spinal cord – below the injury – through electrodes that stimulate the neural pathways that activate leg muscles during natural locomotion.
The neuroprosthetic interface was conceived at EPFL in Lausanne, Switzerland, and developed together with an international network of collaborators including Medtronic, Brown University and Fraunhofer ICT-IMM. It was tested in collaboration with the University of Bordeaux, Motac Neuroscience and the Lausanne University Hospital (CHUV).
“This is the first time that neurotechnology restores locomotion in primates,” says neuroscientist Grégoire Courtine who led the collaboration and holds the IRP chair in spinal cord repair. “But there are many challenges ahead and it may take several years before all the components of this intervention can be tested in people.”

Decoding brain signals and activating leg muscles
The brain is a huge network of cells called neurons. Information is processed in the brain by transmitting spikes of electricity from one neuron to the next. This electrical spiking gives rise to brain signals that can actually be measured and interpreted.
The lumbar region of the spinal cord also contains complex networks of neurons that activate leg muscles to walk. Bundles of nerves coming from the brain carry the relevant information to the spinal cord about the intended activation of leg muscles.
For intact nervous systems, signals about walking come from a small region (about the size of a dime for primates) of the brain called the motor cortex. Signals from the motor cortex travel down the spinal cord, reach the neural networks located in the lumbar region, and these in turn activate muscles in the legs to produce walking movements.
Spinal cord lesions partly or completely prevent these signals from reaching the neurons that activate leg muscles, leading to paralysis. But the motor cortex can still produce spiking activity about walking, and the neural networks activating muscles in the paralyzed leg are still intact and can still generate leg movements.
How the brain-spine interface works
The brain-spine interface bridges the spinal cord injury, in real-time and wirelessly. The neuroprosthetic system decodes spiking activity from the brain’s motor cortex and then relays this information to a system of electrodes located over the surface of the lumbar spinal cord, below the injury. Electrical stimulation of a few volts, delivered at precise locations in the spinal cord, modulates distinct networks of neurons that can activate specific muscles in the legs.
“To implement the brain-spine interface, we developed an implantable, wireless system that operates in real-time and enabled a primate to behave freely, without the constraint of tethered electronics,” says Courtine. “We understood how to extract brain signals that encode flexion and extension movements of the leg with a mathematical algorithm. We then linked the decoded signals to the stimulation of specific hotspots in the spinal cord that induced the walking movement.”
For partial lesions of the spinal cord, the scientists showed that the primate regained control of its paralyzed leg immediately upon activation of the brain-spine interface. The interface should also work for more severe lesions of the spinal cord, according to the scientists, likely with the aid of pharmacological agents. Note that for these partial lesions, the primate is initially paralyzed and then spontaneously regains full mobility after about three months.
“The primate was able to walk immediately once the brain-spine interface was activated. No physiotherapy or training was necessary,” says neuroscientist Erwan Bezard of Bordeaux University who oversaw the primate experiments.
Clinical Trials
“The link between the decoding of the brain and the stimulation of the spinal cord – to make this communication exist – is completely new,” says neurosurgeon Jocelyne Bloch of the Lausanne University Hospital (CHUV) who leads the functional neurosurgery department at the Lausanne University Hospital and surgically implanted the brain and spinal cord implants.
She continues, “For the first time, I can imagine a completely paralyzed patient able to move their legs through this brain-spine interface.”
In collaboration with EPFL, Bloch is currently leading a clinical feasibility study that evaluates the therapeutic potential of this spinal cord stimulation technology, without the brain implant, to improve walking in people with partial spinal cord injury affecting the lower limbs.
Funding Parrtners: European Community's Seventh Framework Program, International Foundation for Research in Paraplegia, European Research Council, The Wyss Centre in Geneva, Marie Curie Fellowship, Marie Curie COFUND EPFL fellowships, Medtronic, Morton Cure Paralysis Fund fellowship, NanoTera.ch Programme (SpineRepair), National Centre of Competence in Research in Robotics, Sinergia program, Sino-Swiss Science and Technology Cooperation, Swiss National Science Foundation.