New ideas for old drugs
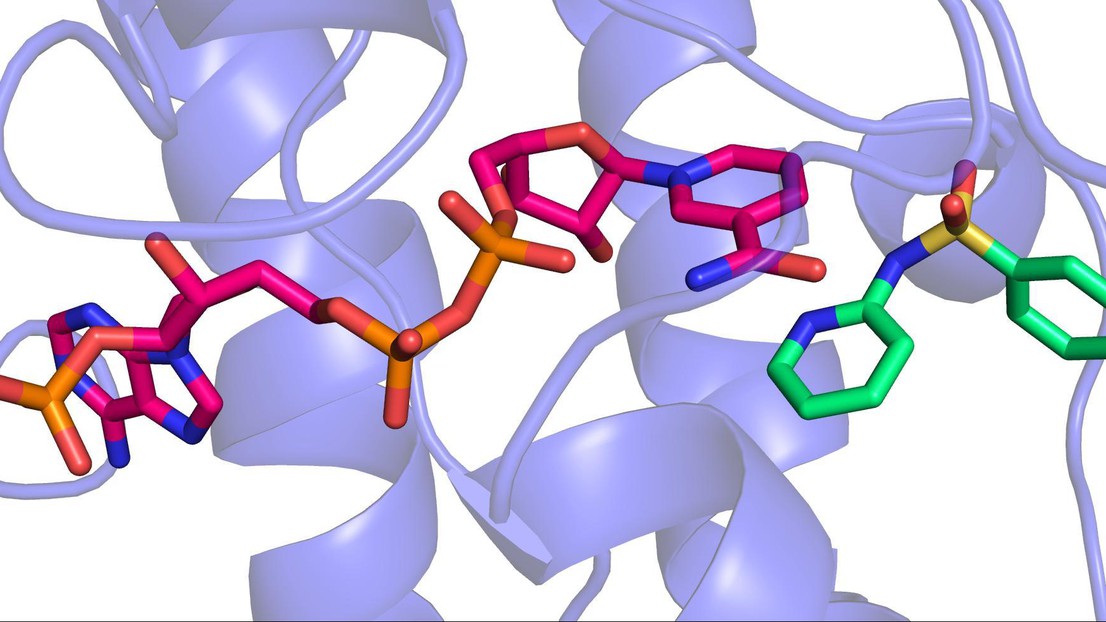
3D image of a sulfonamide binding to the enzyme that makes BH4 © 2013 EPFL
Scientists at EPFL have uncovered the molecular basis behind some of the neurological side effects of sulfonamide antibiotics, providing doctors with possible means to minimize them in patients.
Since the discovery of Prontosil in 1932, sulfonamide antibiotics have been used to combat a wide spectrum of bacterial infections, from acne to chlamydia and pneumonia. However, their side effects can include serious neurological problems like nausea, headache, dizziness, hallucinations and even psychosis. In a recent Science publication, EPFL researchers have shown for the first time how sulfonamides can interfere with a patient’s nervous system.
The problem is that, even though we know how sulfonamides work, we do not understand the actual molecular mechanics behind their side effects. Consequently, it is difficult to modify drug structure or customize therapeutic regimes in order to better serve the needs of patients. That is the critical issue addressed by a team of EPFL scientists led by Kai Johnsson at EPFL’s Laboratory of Protein Engineering (LIP).
The team drew from previous research showing that blocking the activity of a certain enzyme (sepiapterin reductase) affects the levels of an important molecule called tetrahydrobiopterin (BH4) in cells. BH4 is critical for the production of neurotransmitters like serotonin and dopamine, and BH4 deficiency causes similar neurological problems to those associated with sulfonamide side effects.
The EPFL scientists showed for the first time that sulfonamides actually bind to the part of the enzyme that makes BH4. Using a high-throughput drug screening system, the researchers identified ten sulfonamides that strongly inhibit the enzyme. Taking advantage of the expertise of Florence Pojer at EPFL’s Global Health Institute, the scientists were able to solve the enzyme’s molecular structure and determine how sulfonamides bind to it.
Sulfonamides also seem to act on the actual biochemical pathway that synthesizes BH4, as increasing doses of the drugs decreased BH4 concentrations in cultured human cells. The critical finding, however, was that along with BH4, sulfonamides also reduced the actual production of dopamine. By giving cultured human nerve cells different sulfonamides, the researchers found that their natural production of dopamine decreased in proportion to the sulfonamide doses. In addition, it was clear that the impact on dopamine production was different between sulfonamides.
The group’s work shows for the first time that sulfonamides interfere with the biosynthesis of neurotransmitters, which can account for their reported neurological side effects. It also helps us understand how the activity of these drugs relates to their molecular structure, and suggests ways of improving their clinical use.
“Once you know what’s happening you can begin to think about strategies to address the problem – and that is the impact of this work”, says Kai Johnsson. “Historically, I don’t think that there is a more important class of drugs than sulfonamides, and now we can understand them better. It also reminds us that surprising discoveries can be made even for drugs this old.”
---
This project represents a collaboration between EPFL’s School of Basic Sciences and School of Life Sciences, and was further supported by the Swiss National Science Foundation (SNSF) and the Swiss National Centre of Competence in Research (NCCR) Chemical Biology, who also provided the high-throughput drug screening system.