How receptor mobility and function are linked
Heterogeneous diffusion of NK1 receptors in the plasma membrane of living cells © Horst Vogel/EPFL
EPFL scientists have used quantum dots to track individual receptors in the cell membrane. The work connects receptor function to mobility, and offers new insights to regulation of signal transmission in the cell.
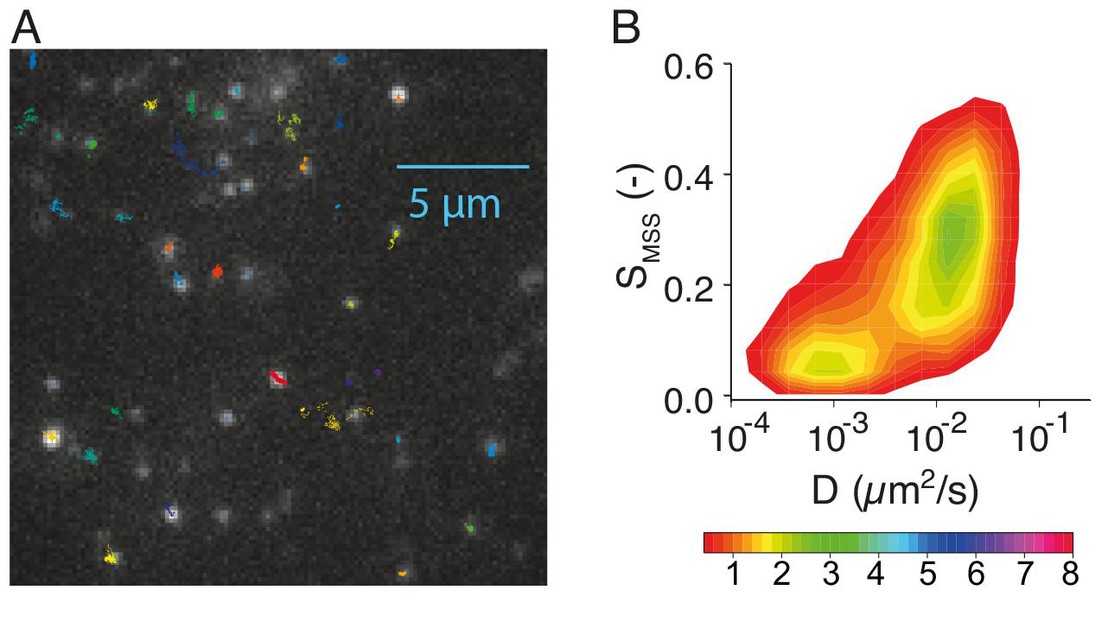
Image caption: (A) Trajectories of single receptors in the plasma membrane of a single cell. (B) Mobility pattern of hundreds of single receptors. S is a measure of receptor confinement and D the the receptor diffusion coefficient in the plasma membrane. The color code scales with the frequency of the individual mobility states.
Lateral diffusion is a process by which proteins and lipids on the cell membrane move side-to-side to interact. Lateral diffusion enables efficient interactions between membrane proteins, which in turn allow signals to be transmitted across the plasma membrane. However, how the spatio-temporal distribution of cell-surface receptors, such as G protein-coupled receptors (GPCRs), influences the cellular signaling network is still unknown. Using quantum dots, EPFL scientists have now studied the mobility of an “archetype” GPCR, and have discovered a heterogeneous mobility distribution pattern, which is directly linked to the receptor’s function. The study is published in the Journal of Biological Chemistry.
A team led by Horst Vogel at EPFL studied the neurokinin-1 receptor, a GPCR, during its different phases of cell signaling. The researchers attached a single quantum dot to individual receptors in live cells. The technique allowed them to track their mobility in cell membranes with high spatial and temporal resolution over long time regimes.
The receptors showed broad, heterogeneous mobility distribution patterns depending on the signaling phase they were contributing to. Diffusion constants ranged between 0.0005 to 0.1 μm2/s for both freely moving receptors, those confined in specific membrane domains, and immobile ones.
The scientists found that receptors fell into two major, broadly distributed receptor populations. One showed high mobility but low lateral restriction; the other population showed low mobility but high restriction. In fact, about 40% of the receptors were already confined in membrane domains, and are associated with the protein clathrin, which plays a major role in the formation of coated vesicles in the cell, e.g. for endocytosis.
By activating and inhibiting clathrin in the cells, the scientists determined that the receptors confinement depends on the amount of membrane-associated clathtrin present. Confinement was also found to correlate with a significant decrease of normal receptor activity.
The work shows that this high plasticity, or heterogeneity, of receptor mobility plays a major role in regulating both receptor activity and homeostasis. The findings offer new insights to the regulation of receptor signaling in general.
This work was funded by EPFL, the Swiss National Science Foundation, the National Centre of Competence in Research (NCCR) Chemical Biology, and the European Community (Project SynSignal).
Reference
Veya L, Piguet J, Vogel H. Single-Molecule Imaging Deciphers the Relation between Mobility and Signaling of a Prototypical G Protein-Coupled Receptor in Living Cells. JBC 290(46): 27723-27735, November 13, 2015. DOI: 10.1074/jbc.M115.666677