ChemMedChem Volume 7, Issue 7, pages 1173–1176, July 2012.
© 2012 EPFL
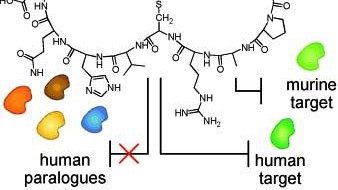
Bicyclic Peptides with Optimized Ring Size Inhibit Human Plasma Kallikrein and its Orthologues While Sparing Paralogous Proteases.
 New drug candidates require testing in animal models prior to approval for clinical use. A recently developed antagonist based on a bicyclic peptide inhibited the human serine protease plasma kallikrein potently and selectively. However, the inhibitor was ‘too’ selective, not inhibiting mouse plasma kallikrein which prevented its testing in animal models. Researchers led by Prof. Christian Heinis (Laboratory of Therapeutic Proteins and Peptides) have developed bicyclic peptides that inhibit both human and murine plasma kallikrein, but not any paralogous proteases; their work is reported in ChemMedChem and highlighted on the cover of the same journal as well as by the life-sciences news portal Bionity.com.
New drug candidates require testing in animal models prior to approval for clinical use. A recently developed antagonist based on a bicyclic peptide inhibited the human serine protease plasma kallikrein potently and selectively. However, the inhibitor was ‘too’ selective, not inhibiting mouse plasma kallikrein which prevented its testing in animal models. Researchers led by Prof. Christian Heinis (Laboratory of Therapeutic Proteins and Peptides) have developed bicyclic peptides that inhibit both human and murine plasma kallikrein, but not any paralogous proteases; their work is reported in ChemMedChem and highlighted on the cover of the same journal as well as by the life-sciences news portal Bionity.com.
Vanessa Baeriswyl et al., ChemMedChem, Volume 7, Issue 7, pages 1173–1176, July 2012