A molecular lid caps the growth of centriolar microtubules
© N.J. Dynes/P. Gönczy (EPFL)
Working with researchers at the PSI and at the University of Utrecht, EPFL scientists uncover how a protein acts as a molecular "lid" to cap microtubules, thus regulating the length of centrioles.
Centrioles are cylindrical microtubule-based cellular organelles. Centrioles form cilia, which are important for signaling and cell motility, as well as centrosomes, which organize microtubules in the cytoplasm. Microtubules that are part of the centriole grow at significantly slower rates than those present in the cytoplasm, which has been a mystery to cell biologists. Publishing in Developmental Cell, scientists have now discovered that a protein called centrosomal P4.1-associated protein (CPAP) appears key in imparting this difference by acting as a “lid” that caps microtubule growth at the centriole.
The lab of Pierre Gönczy at EPFL, working with colleagues at the Paul Scherrer Institute and at the University of Utrecht combined structural biology, in vitro reconstitution assays, and cell biology to investigate the role of CPAP in regulating the growth of centriole microtubules. The protein, whose essential role in centriole formation was initially discovered in the nematode C. elegans in Gönczy’s lab, is one of several proteins that interact directly with centriolar microtubules.
Microtubules are long tube-like polymers, made from subunits of a/b tubulin dimers. This study shows that CPAP binds and "caps" the growing (plus) end of microtubules, where tubulin binding sites are exposed for interactions with incoming tubulin dimers. Using X-ray crystallography and biophysical assays, the team found that CPAP associates with a site on the tubulin dimer that is involved in longitudinal tubulin‐tubulin interactions critical for microtubule growth. Thus, interaction of CPAP with this site could interfere with the addition of new tubulin subunits to the polymer.
Accordingly, the study found that CPAP slows the growth of microtubules in vitro and stabilizes them by inhibiting so-called microtubule “catastrophes” and promoting “rescues”.
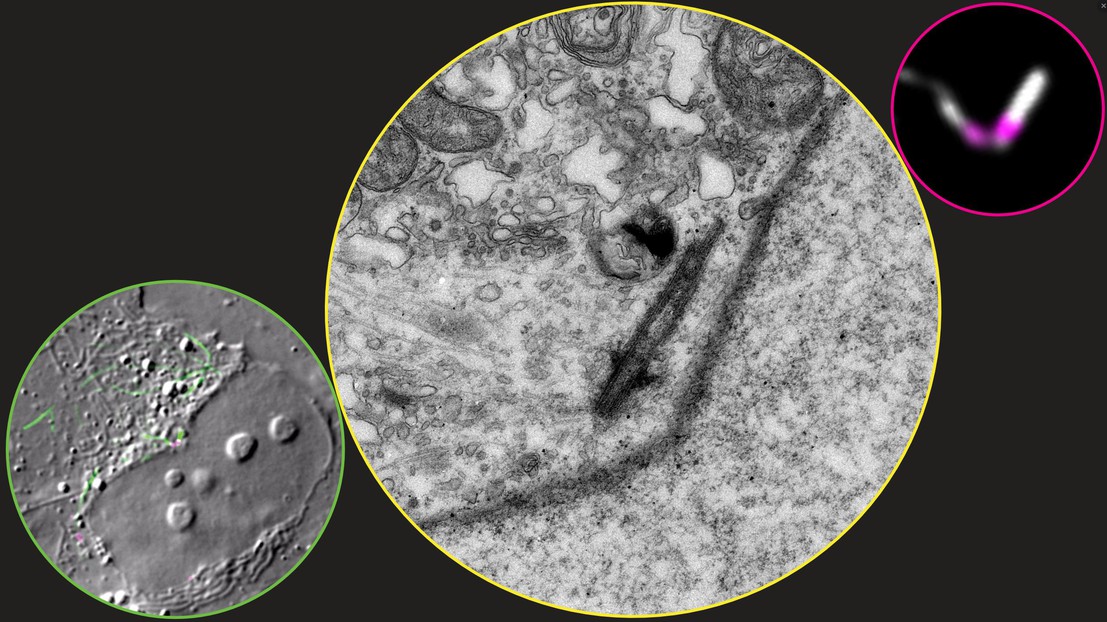
Furthermore, the work shows that expression of CPAP without the “lid” function in human cells leads to abnormally long fibers emanating from the centrioles. Collaboration with the Bio-EM facility at EPFL allowed the researchers to determine that these fibers were actually extensions of the centriolar microtubules.
Overall, the study demonstrates how CPAP, by acting as a molecular "lid", participates in ensuring the slow assembly of centriolar microtubules, thus contributing to the control of organelle length in the cell.
---
Image caption: DIC and fluorescence (left), electron microscopy (middle), and fluorescence (right) images of the same human cell expressing a YFP-CPAP mutant lacking the LID domain (YFP-CPAP ΔLID, white in fluorescence image). The fluorescence image shows a pair of centrioles, marked with tagRFP-Centrin 1 (magenta) with long YFP-CPAP ΔLID (white) fiber, several fold longer than a normal centriole. Electron microscopy (single section from serial section series) reveals that this fiber is a continuation of the centriolar microtubules, protruding from the distal end of the centriole and reaching a total length of 1.6 μm (compared to the normal 0.5 μm). The other centriole and its extension are not visible in this particular EM section.
---
This work represents an equal collaboration between the Swiss Institute for Experimental Cancer Research (EPFL) with PSI’s Laboratory of Biomolecular Research, the Faculty of Science of Utrecht University, and EPFL’s Bio-EM Facility. It was funded by the European Molecular Biology Organization (EMBO), the Swiss National Science Foundation, and the European Research Council.
Reference
Sharma A, Aher A, Dynes NJ, Frey D, Katrukha EA, Jaussi R, Grigoriev I, Croisier M, Kammerer RA, Akhmanova A, Gönczy P, Steinmetz MO. Centriolar CPAP/SAS‐4 imparts slow processive microtubule growth. Developmental Cell 23 May 2016. DOI: 10.1016/j.devcel.2016.04.024