A cost-effective and energy-efficient approach to carbon capture
© 2014 EPFL Jamani Caillet
Scientists from EPFL, UC Berkeley and Beijing have developed a slurry-based process that can revolutionize carbon capture. The slurry, consisting of a porous powder suspended in glycol, offers the efficient large-scale implementation of a liquid while maintaining the lower costs and energy efficiency of solid carbon-capturing materials.
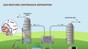
Carbon capture is a process by which waste carbon dioxide (CO2) released by factories and power plants is collected and stored away, in order to reduce global carbon emissions. There are two major ways of carbon capture today, one using powder-like solid materials which “stick” to CO2, and one using liquids that absorb it. Despite their potential environmental and energy benefits, current carbon capture strategies are prohibitive because of engineering demands, cost and overall energy-efficiency. Collaborating scientists from EPFL, UC Berkeley and Beijing have combined carbon-capturing solids and liquids to develop a “slurry” that offers the best of both worlds: as a liquid it is relatively simple to implement on a large scale, while it maintains the lower costs and energy efficiency of a solid carbon-capturing material. The breakthrough method is published in Nature Communications.
The most common approach to carbon capture uses liquid amine solutions, which can absorb CO2 from the atmosphere. On a large scale, the system uses two columns, one for capturing CO2 and the other for releasing it from the liquid, in a process referred to as “regeneration”. For amine solutions, regeneration is the most energy-consuming part because the CO2 is so strongly bound to the amine molecules that it is necessary to actually boil them in order to separate them.
An alternative to liquids is to use solid materials known as “metal-organic frameworks” (MOFs). These are fine powders whose particles are made up of metal atoms that are connected into a 3D structure with organic linkers. Their surface is covered with nano-size pores that collect CO2 molecules. But despite its lower cost, as this method involves transporting solids it is very demanding in terms of engineering. Berend Smit, Director of the Energy Center at EPFL, explains: “Imagine trying to walk with a plateful of baby powder. It’s going to go everywhere, and it’s very difficult to control.”
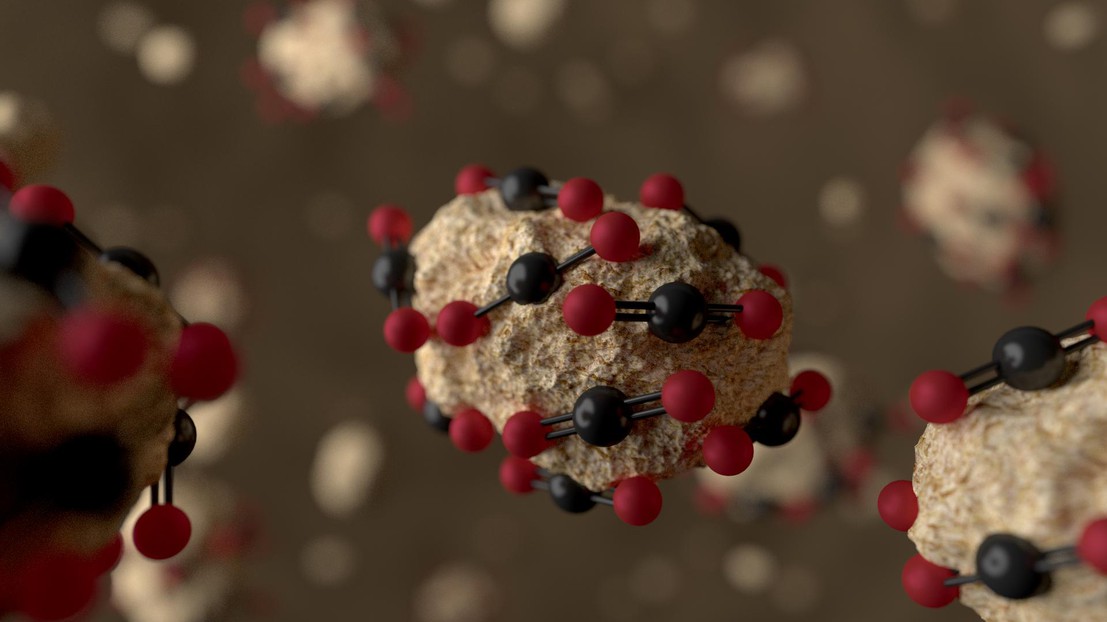
Working with scientists from Beijing and UC Berkeley, Smit is a lead author on a breakthrough carbon-capture innovation that uses a mixture of solid and liquid in solution called a “slurry”. The solid part of the slurry is a MOF called ZIF-8, which is suspended in a 2-methylimidazole glycol liquid mixture.
“Why a slurry?” says Smit. “Because in the materials that are currently used for adsorption the pores are too large and the surrounding liquid would fill them, and not let them capture CO2 molecules. So here we looked at a material – ZIF-8 – whose pores are too small for the glycol’s molecules to fit, but big enough for capturing the CO2 molecules from flue gas.”
ZIF-8 is a good material for carbon-capturing slurries, because it displays excellent solution, chemical and thermal stability, which is important for repeated regeneration cycles. ZIF-8 crystals have narrow pores (3.4 Å in diameter) that are smaller than the diameter of glycol molecules (4.5 Å), preventing them from entering. Even though other liquids were tested in the design of the slurry, including ethanol, hexane, methylbenzene and tetrachloromethane, their molecules are small enough to enter the ZIF-8 pores and reduce its carbon capturing efficiency. In this respect, glycerol has so far been shown to be an ideal liquid.
The concept of the slurry comes from an idea of one of Smit’s former PhD students who is now a professor in Beijing, and it could be the key to large-scale implementation of carbon capture. “Pumping slurry is much easier than transporting a pile of baby powder,” says Smit. “And we can use the same technologies for heat integration as the liquid process.”
Because it combines the low cost and efficiency of nano-porous materials with the ease of a liquid-based separation process, the slurry successfully addresses these two main obstacles to the implementation of carbon capture in the real world. In addition, it shows exceptionally good separation from CO2, meaning that it doesn’t require excessive amounts of energy (e.g. boiling) in order to regenerate, which increases its overall energy efficiency.
The slurry offers a new template for developing similar combinations in the future. Following their successful proof-of-concept work, the research teams are now planning to test the ZIF-8/glycol slurry in the field.
This work represents a collaboration between EPFL, China University of Petroleum, University of California, Berkeley and Beijing University of Chemical Technology.
Reference
Liu H, Liu B, Lin L-C, Chen G, Wu Y, Wang J, Gao X, Lv Y, Pan Y, Zhang X, Zhang X, Yang L, Sun C, Smit B, Wang W. A hybrid absorption–adsorption method to efficiently capture carbon.Nature Communications DOI: 10.1038/ncomms6147